์ธ์ฆ ๋ด์ญ
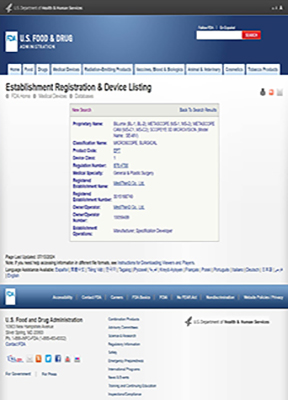
๋ฏธ๊ตญ
FDA
Class1
SCOPEYE, METASCOPE, SCOPEYE 3D MICROVISION, SCOPEYE 3D ADDON
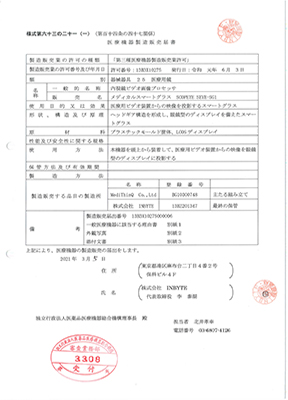
์ผ๋ณธ
PMDA
Class1
METASCOPE, SCOPEYE

์ ๋ฝ
CMC
CE MDR Class1
SCOPEYE, METASCOPE, SCOPEYE 3D MICROVISION, SCOPEYE 3D ADDON

์ค์์ค
CMC
Swissmedic Class1
SCOPEYE, SCOPEYE 3D MICROVISION, SCOPEYE 3D ADDON

์๊ตญ
CMC
PARD Class1
SCOPEYE, SCOPEYE 3D MICROVISION, SCOPEYE 3D ADDON

ํธ์ฃผ
TGA
Class1
SCOPEYE

๋๋ง
TFDA
TFDA Class1
SCOPEYE

๋ํ๋ฏผ๊ตญ
DNV
ISO13485:2016
SCOPEYE
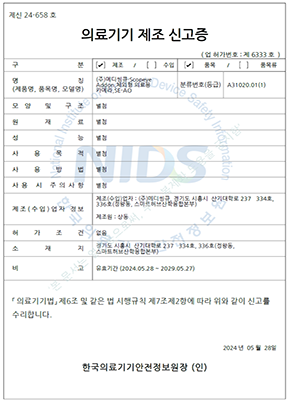
๋ํ๋ฏผ๊ตญ
ํ๊ตญ์์ฝ์ฒ
1๋ฑ๊ธ ์๋ฃ๊ธฐ๊ธฐ
SCOPEYE
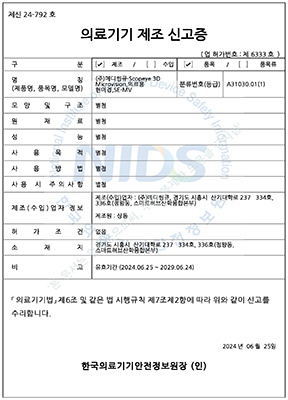
๋ํ๋ฏผ๊ตญ
ํ๊ตญ์์ฝ์ฒ
1๋ฑ๊ธ ์๋ฃ๊ธฐ๊ธฐ
METASCOPE
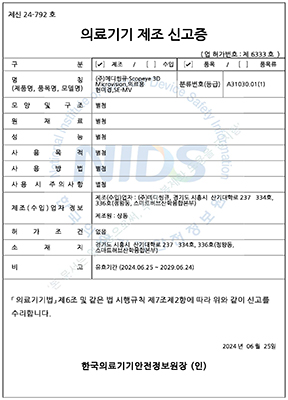
๋ํ๋ฏผ๊ตญ
ํ๊ตญ์์ฝ์ฒ
1๋ฑ๊ธ ์๋ฃ๊ธฐ๊ธฐ
SCOPEYE 3D ADDON
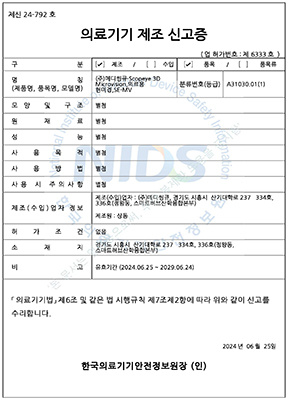
๋ํ๋ฏผ๊ตญ
ํ๊ตญ์์ฝ์ฒ
1๋ฑ๊ธ ์๋ฃ๊ธฐ๊ธฐ
SCOPEYE 3D MICROVISION